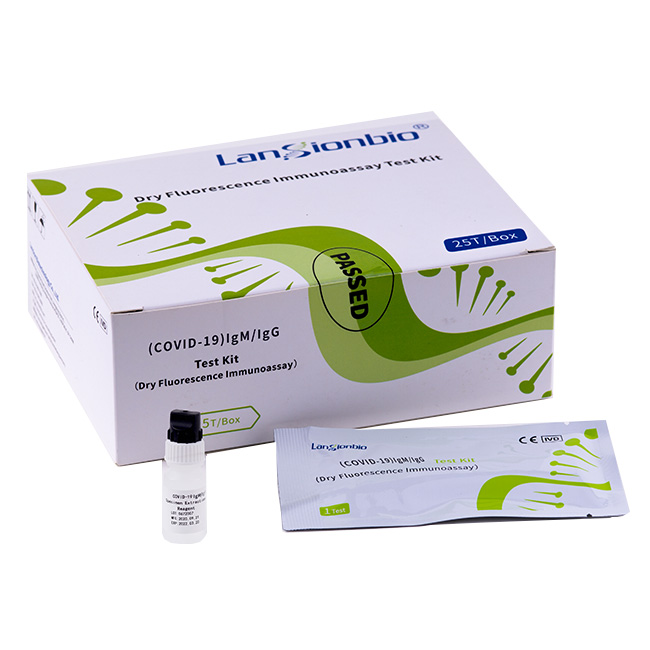
(COVID-19) IgM/IgG Test Kit (Dry Fluorescence Immunoassay)
Product Description
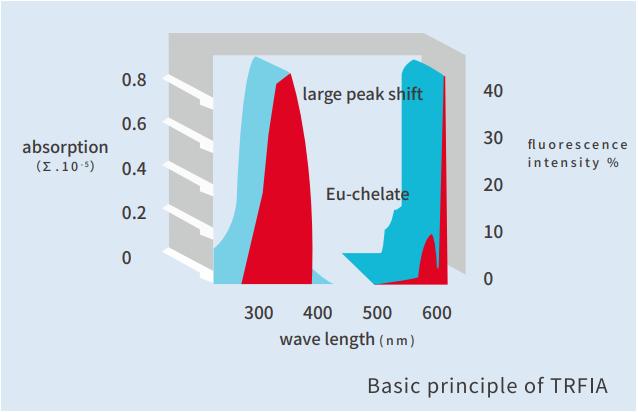
1. DSC utilizes an advanced methodology called Time-resolved Fluorescence Immunoassay (TRFIA) Method. TRFIA is super-sensitive detection technique characterized by specific fluorescence of rare earth ions. It is not only highly sensitive, but also overcomes the instability of enzyme marker and is the best choice for immunological detection. The high fluorescence intensity and long life of labeled ionic chelates are beneficial to eliminate the influence of fluorescent substances in samples and environment on the test results.
2. Analyzer Introduction: Analyzer uses the advanced method of Time-resolved Fluorescence Immunoassay (TRFIA), for the in-vitro quantitative detection of bio-markers for Diabetes Mellitus, Inflammation, Cardiovascular Diseases, Hormone, Gastric Diseases, Renal Diseases, Tumor, etc.
3. Application: Laboratory, ER, Cardiology, ICU, Respiratory, Pediatrics, etc.
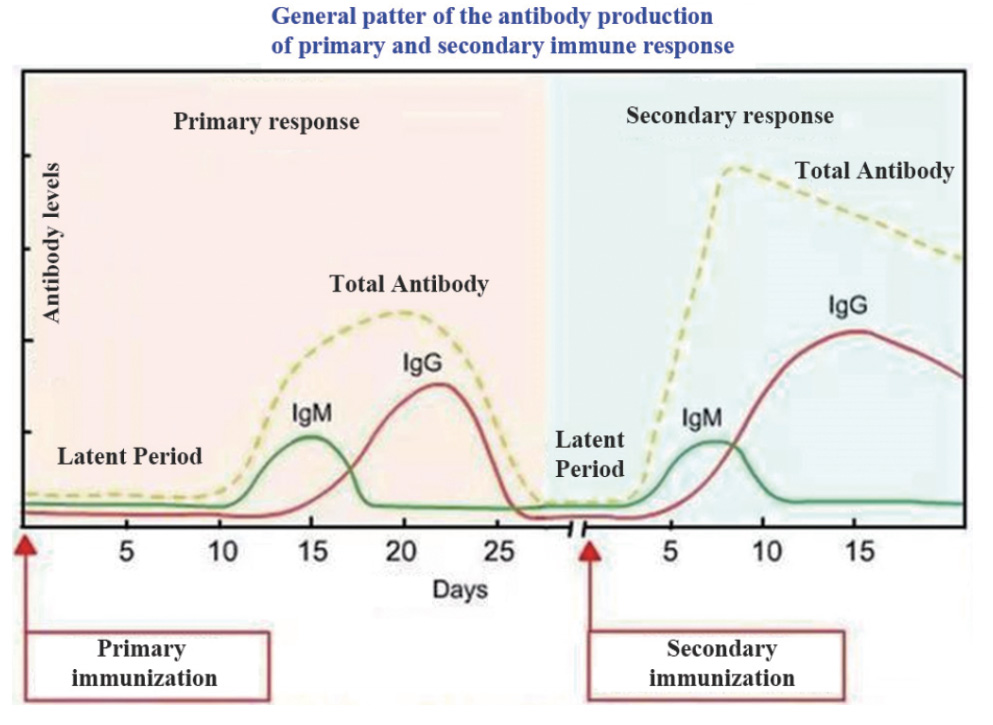
IgM is the antibody occurred in the acute infection stage. IgM can be tested about 3 days after the onset of the disease and disappear within 1 to 2 months after the infection.
IgG is the antibody occurred in the middle and later stage of infection. Positive results indicate that the patient is recovering or has a previous infection. It is used to dynamic monitoring of the process of infection clinically through simultaneous test of IgM/IgG.
Application
Suspected case test in epidemic area;
Early test of fever clinic and CDC;
Screening of primary medical institutions;
Inspection of floating population in railway stations and airports.
Features
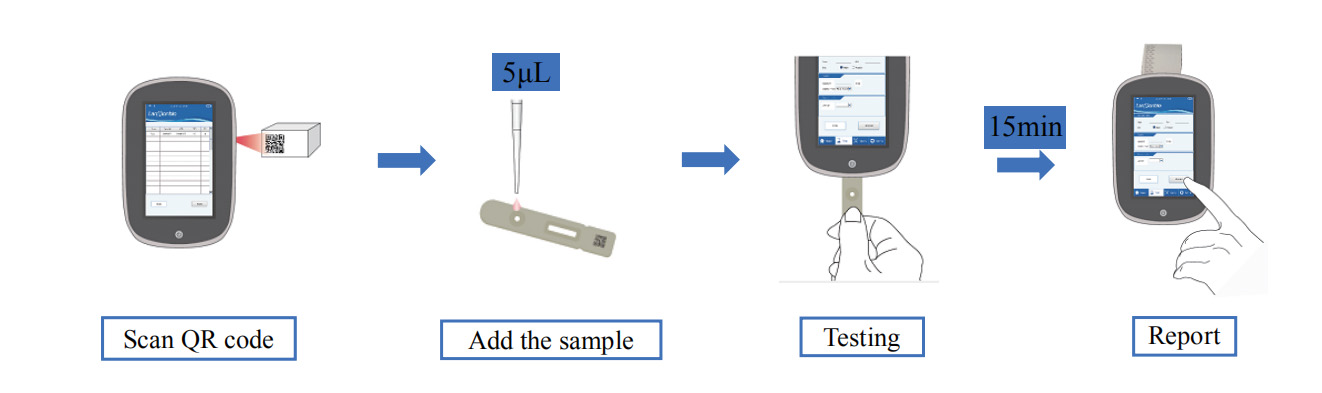
15 minutes
5uL of sample, identify suspected patients quickly.
Accurate result
Time-resolved Fluorescence Immunoassay, high sensitive, screen at early infection.
Reduce the rate of missed diagnosis and diagnosis of nucleic acid testing
Indicate both recent infection and previous infection to reduce missed diagnosis rate.
Combined with nucleic acid testing to improve confirmed rate of suspected patients.
Convenient operation and prevent contamination
Low laboratory requirements, compatible with whole blood/fingertip blood/serum/plasma.
Helpful for early diagnosis and exclusion of suspicious cases.
Suitable for large-scale screening in primary hospitals.
Easy Operation
Product information
| Name | Methodology | Sample Type | Storage and Validity | Specifications |
| (COVID-19) IgM/IgG Test Kit (Dry Fluorescence Immunoassay) |
Time-resolved Fluorescence Immunoassay |
Serum Plasma |
Store at 4-30℃ Within 18 months. |
25 tests/kit 50 tests/kit |