SARS-COV-2 Neutralizing Antibody Test Kit(LFIA) 1PCS/BOX
Intended use
The SARS-CoV-2 Neutralizing antibody Test Kit(LFIA) is suitable for in vitro qualitative detection of SARS-CoV-2 neutralizing antibodies in human serum, plasma, or whole blood samples (capillary or venous) including samples prepared by commonly-used anticoagulants (K2EDTA, Na Citrate, Li-Heparin) from individuals with vaccine injection people and recovered people. SARS-CoV-2 Neutralizing antibody can bind to the pathogenic target proteins RBD and NTD on the surface of the virus, thereby preventing the virus from binding to cell surface receptors. The development of a vaccine depends on whether the neutralizing antibody is produced by immunization, so the detection of the neutralizing antibody is crucial to assess the effectiveness of the vaccine. SARS-CoV-2 Neutralizing antibody Test Kit(LFIA) can quickly and accurately detect neutralizing antibodies, which is of great signifcance for the development of COVID- 19 vaccines, evaluation of effectiveness, and evaluation of neutralizing antibody levels in the population.
Summary
With the global pandemic of the novel coronavirus disease (COVID-19), the number of infections and deaths continues to rise, and scientists from various countries are constantly looking for treatments for COVID-19. For the prevention of sudden major infectious diseases, neutralizing antibody therapy is an important strategy for effective prevention and treatment. Neutralizing antibodies are a kind of soluble protein secreted by adaptive immune response cells, it can recognize the virus surface protein and prevent it from binding to cell receptors. After the virus invades the human body, immune cells secrete neutralizing proteins into the blood. These antibodies prevent the virus from infecting cells by binding to the spike protein on the surface of the virus. Neutralizing Antibodies are expected to become a sharp edge against the novel corona virus disease.
Contents of the Kit
One test kit contains: Test Cassettes |1 Buffer Solution Bottle | 1 Package Insert
Type I test cassette contains
● A test strip in a plastic cassette
● Dried reagents with stabilizers
● Colloidal gold-labeled recombinant SARS-CoV-2 protein NTD
● Colloidal gold-labeled mouse lIgG
● Goat anti-mouse polyclonal antibody
● Mouse anti-human lgG monoclonal antibody
Type II test cassette contains
● A test strip in a plastic cassette
● Dried reagents with stabilizers
● Colloidal gold-labeled recombinant SARS-CoV-2 protein RBD
● Colloidal gold-labeled mouse lgG
● Goat anti-mouse lgG polyclonal antibody
● cMouse anti-human IgG monoclonal antibody
Type III test cassette contains
● A test strip in a plastic cassette
● Dried reagents with stabilizers
● Colloidal gold-labeled recombinant SARS-CoV-2 protein NTD
● Colloidal gold-labeled recombinant SARS-CoV-2 protein RBD
● Colloidal gold-labeled mouse lgG
● Goat anti-mouse polyclonal antibody
● Mouse anti-human IgG monoclonal antibody
Warnings and Precautions
● For human in vitro clinical diagnostics only.
● The product should only be used by healthcare professionals or trained technicians.
● After opening the sealed cassette pouch the test should be used within one hour.
● Do not immerse test cassette in water
● Do not freeze test cassette or buffer solution.
● Handle specimens in accordance to the OSHA Standard on Bloodborne Pathogens.
● Wear protective gloves, clothing, and eyewear.
● Wash hands thoroughly after handling specimens.
● Dispose of all used or damaged test cassettes, buffer solution bottle or other kit components as biohazardous materials.
● Do not use test cassette, buffer solution, or any other kit components if the pouch is damaged orthe seal is broken.
● Do not use samples containing lipids, hemolysis, or turbidity which can affect results.
● This package insert must be read completely before performing the test. Failure to follow directionsin package insert may yield inaccurate test results.
● Test results should be read bet ween 15 and 20 rninutes after s specimen is ap plied to the sammiple well. Results read after 20 minutes may give erroneous results.
● Do not use test cassette, buffer solution, or any kit component beyond the indicated expiration
date.
● Bring all reagents to room temperature before LIse.
Introduction of products
1. Packing size:1test/kit 20test/kit
2. Specimen type:blood/serum/plasma
3. Storage:2-30℃
4. Shelf life:24 months
New method by (LFIA)
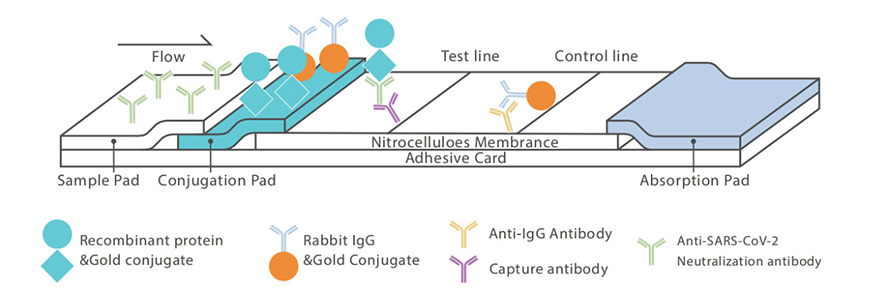
Testing methods for SARS -Cov-2
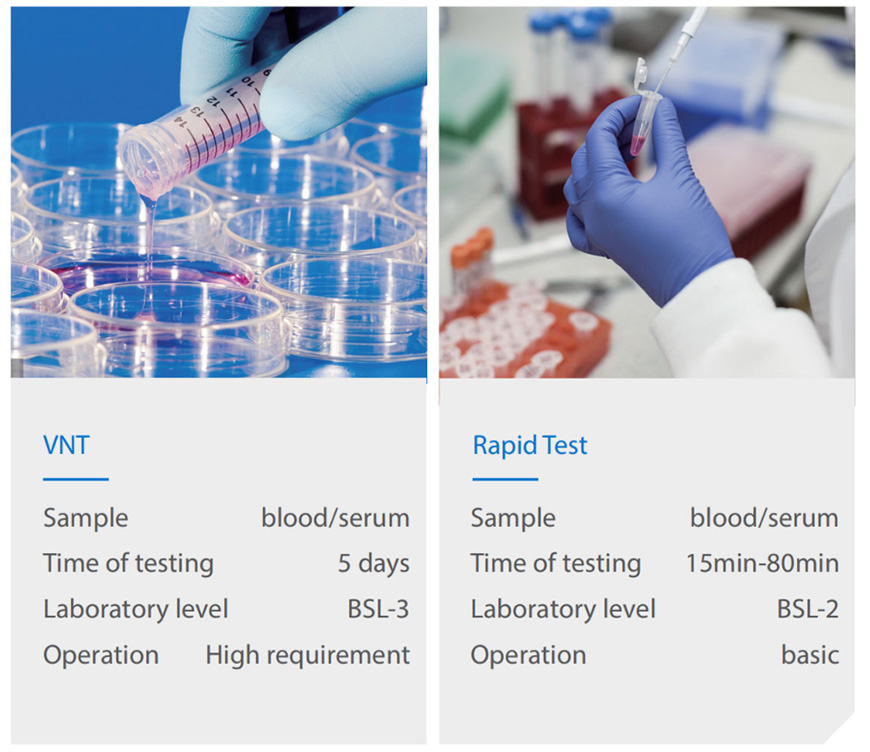
How does the virus infect the cell
For the virus to invade the human cells, it needs to attach to the ACE2 receptor with the receptor binding domain (RBD) of the Spike protein.
If this interaction is blocke by a Neutralizing/blocking an-tibody, the virus can not invade the cell.








